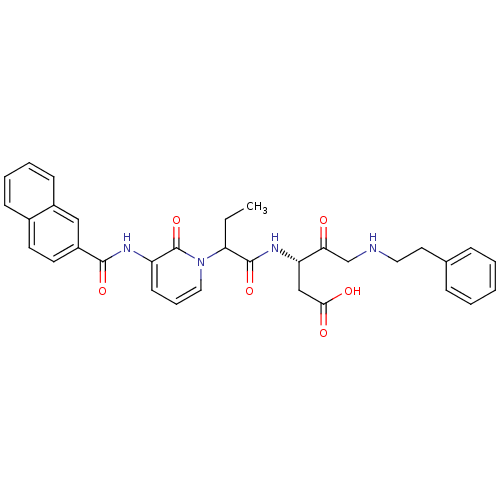
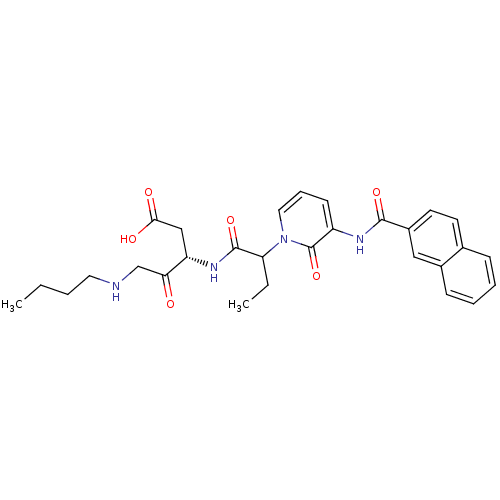
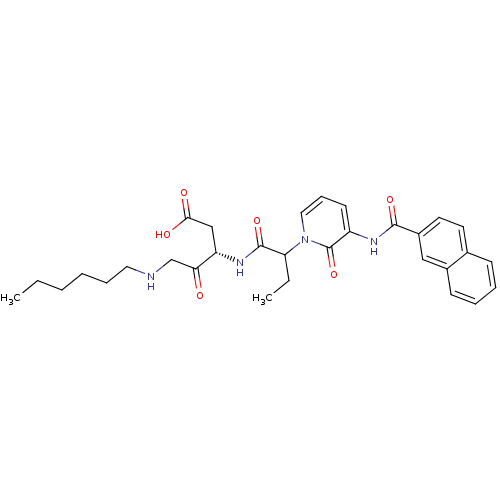
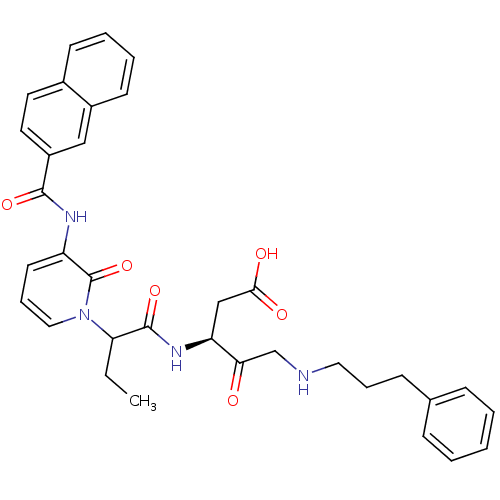
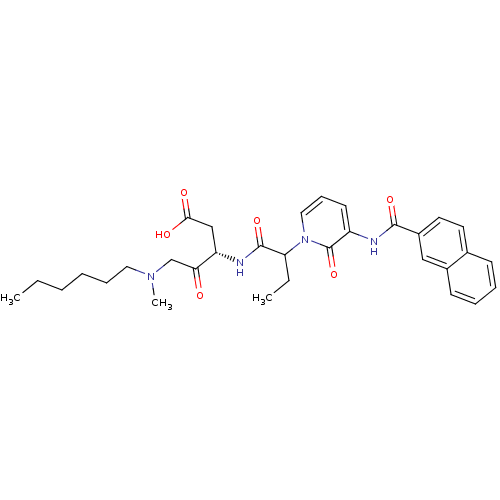
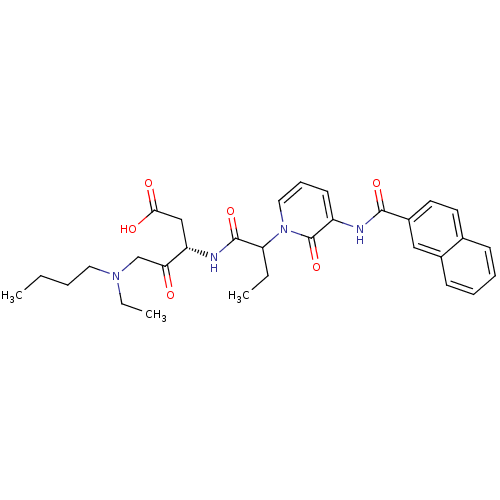
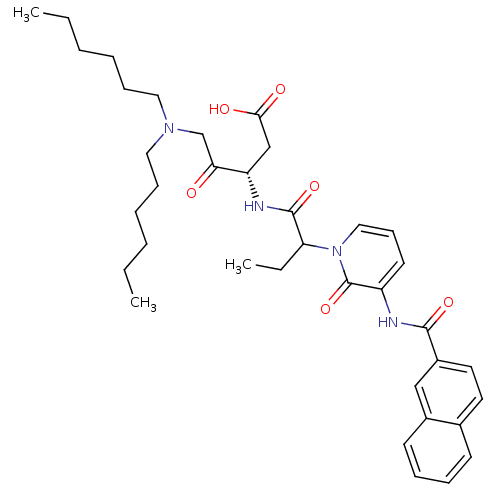
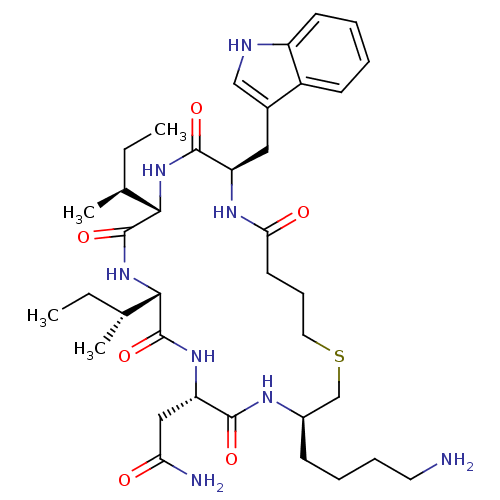
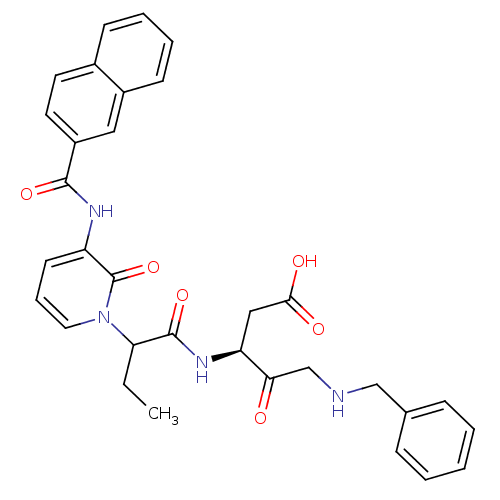
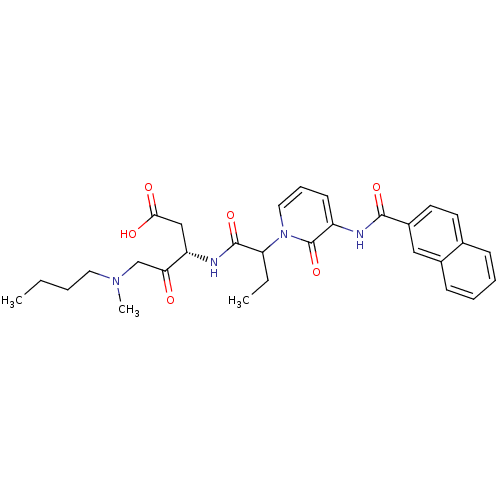
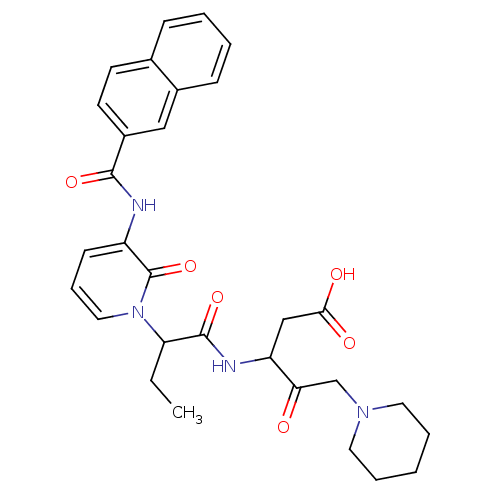
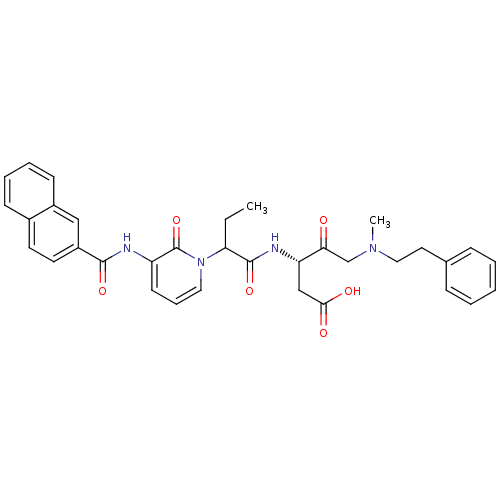
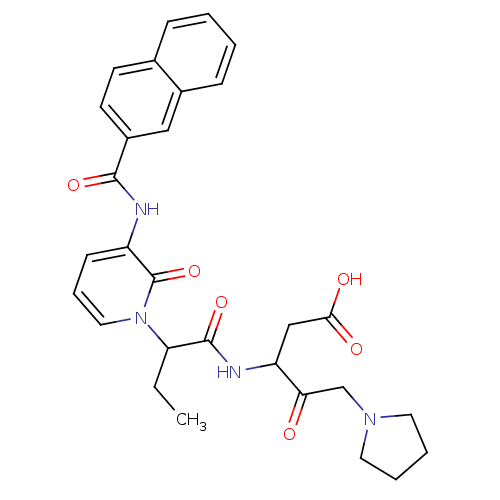
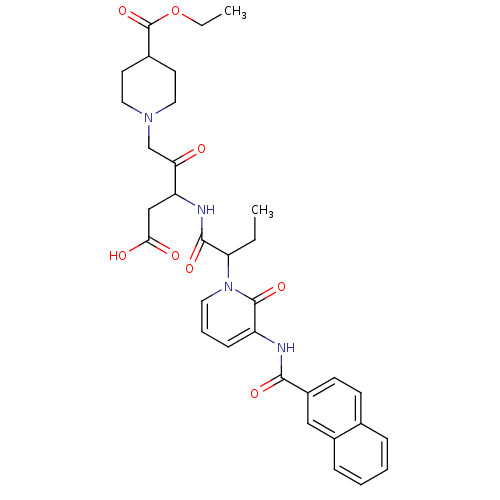
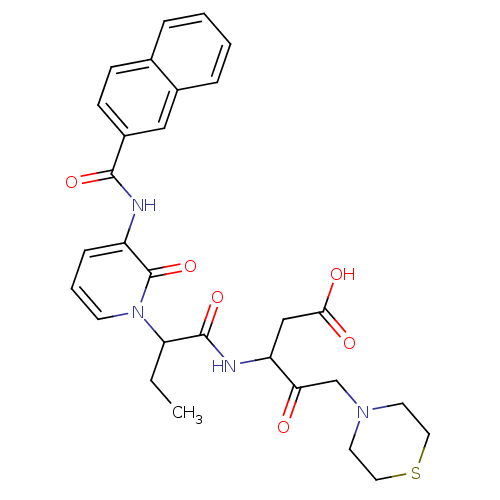
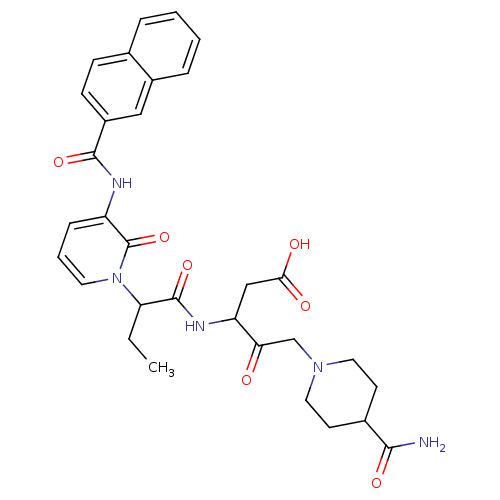
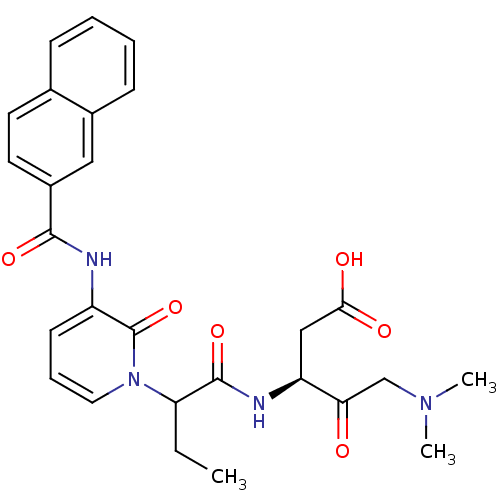
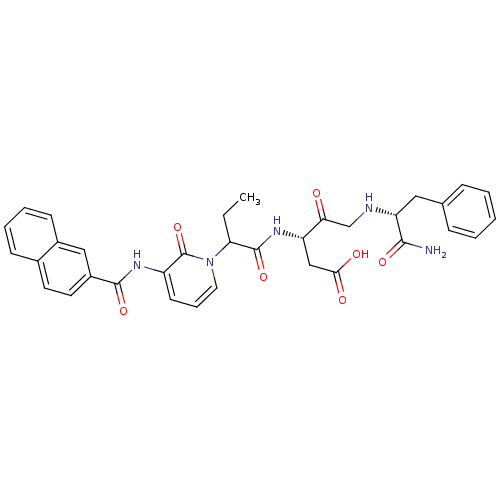
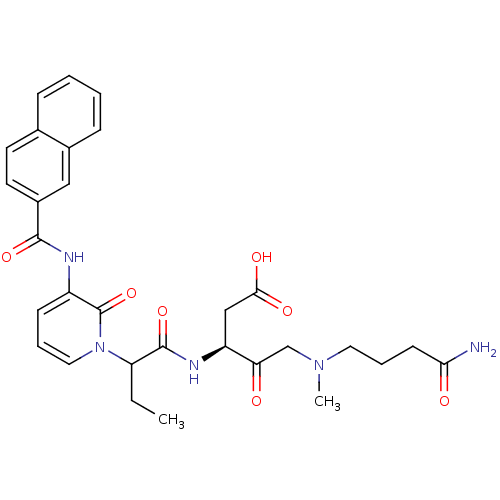
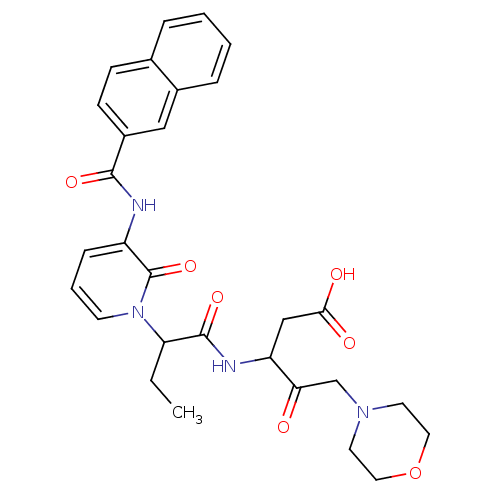
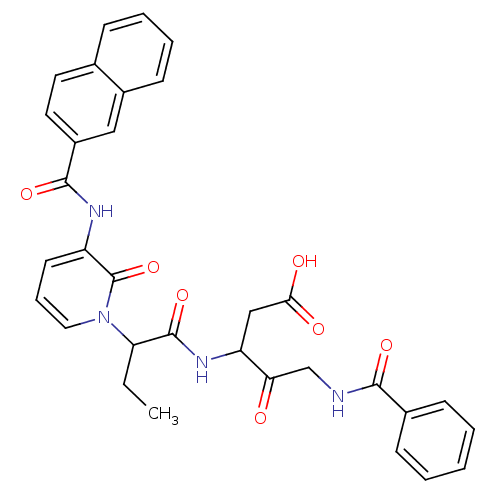
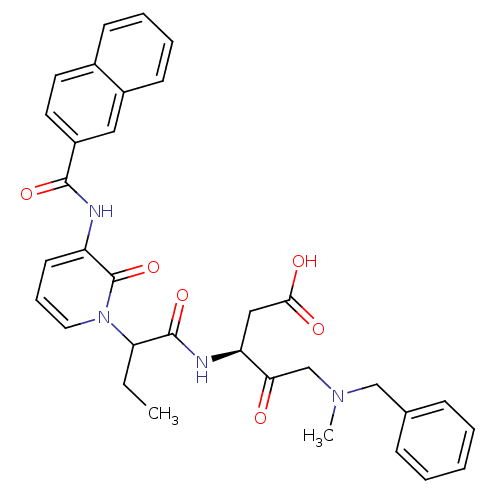
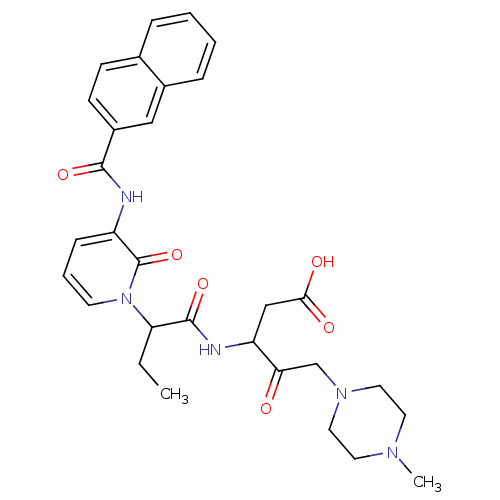
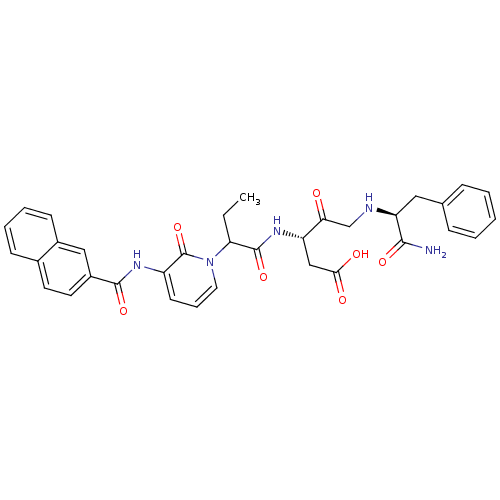
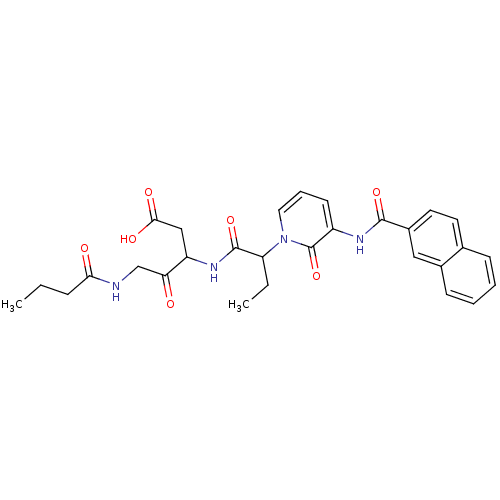
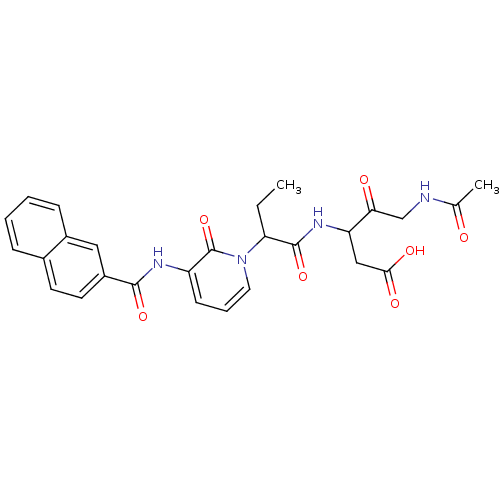
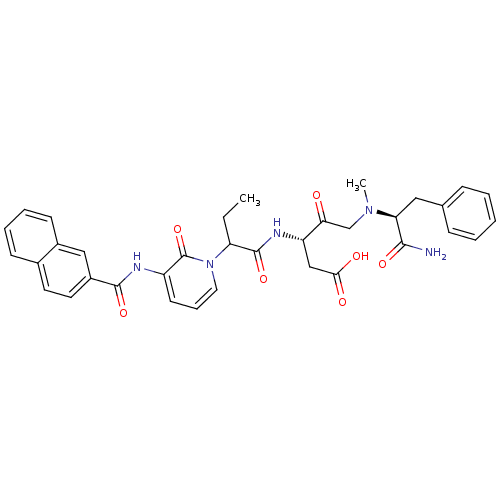
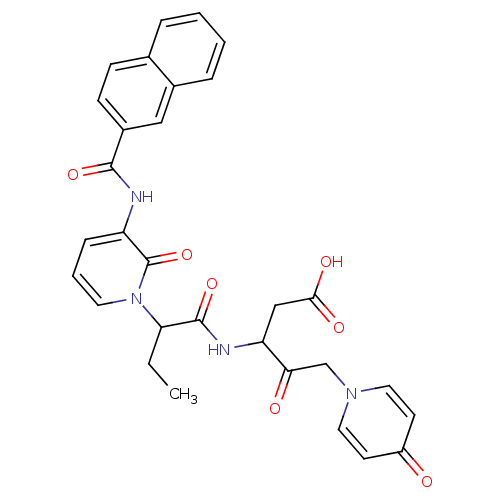
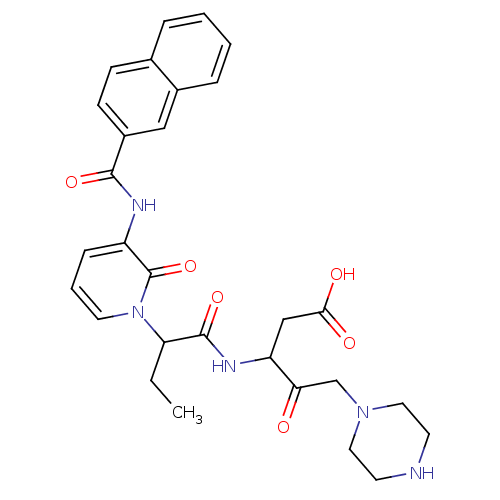
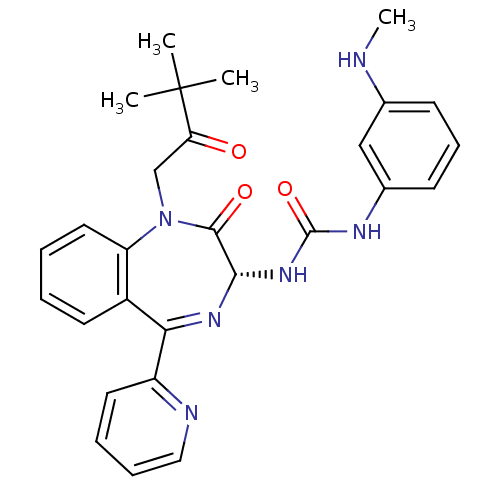
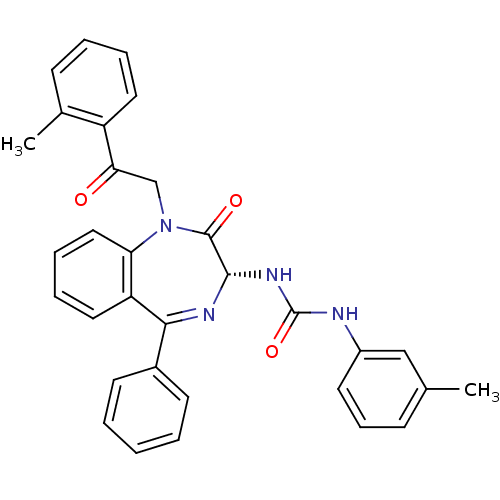
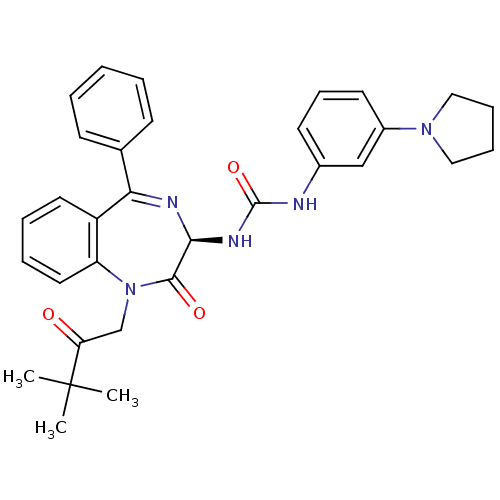
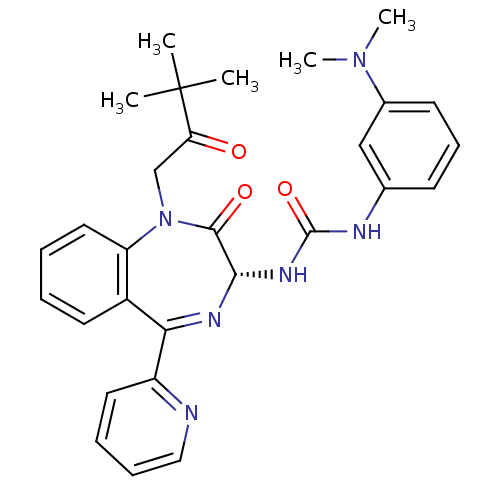
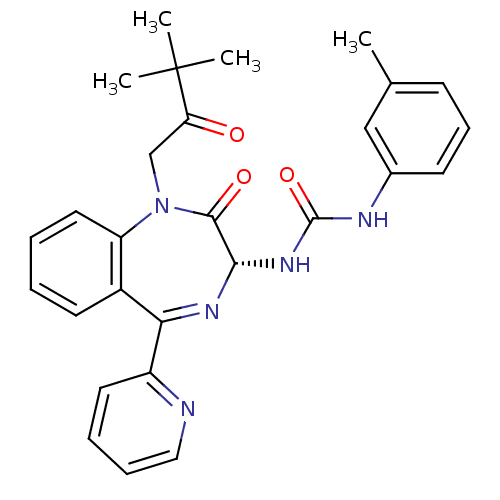
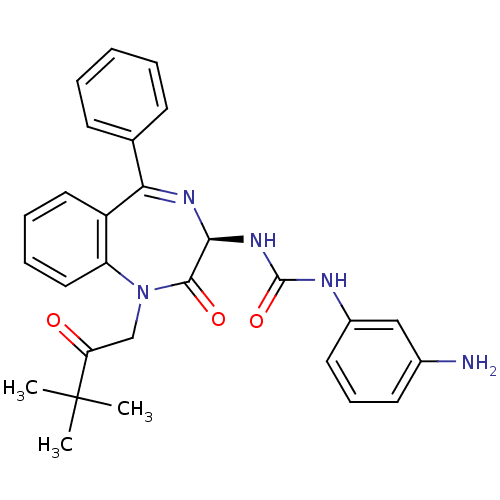
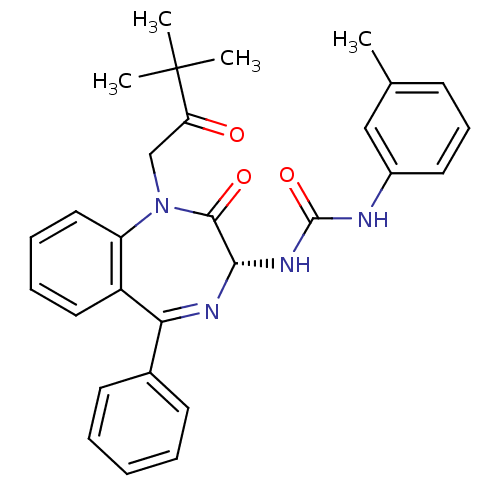
Affinity DataKi: 0.370nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 0.760nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 0.850nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:In vivo antagonistic activity against cloned human oxytocin receptor overexpressed in a stable HEK293 cell lineMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 7.30nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:In vivo antagonistic activity against cloned human oxytocin receptor overexpressed in a stable HEK293 cell lineMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:In vivo antagonistic activity against cloned human oxytocin receptor over-expressed in a stable HEK293 cell lineMore data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 58nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 58nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 78nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 81nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 117nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 255nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 395nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:In vivo antagonistic activity against cloned human oxytocin receptor overexpressed in a stable HEK293 cell lineMore data for this Ligand-Target Pair
Affinity DataKi: 1.65E+3nMAssay Description:Inhibition of human recombinant IL-1 beta converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.520nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.840nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair